| Chemical Name | Amino Sulphonic Acid |
|---|---|
| Chemical Formula | NH2SO3H |
| Molecular Weight | 97.09 |
| Sulphamic acid content | 98%(min.) |
| Moistuer | 1-2% (max.) |
| Physical Appearance | White Powder |
| Matter Insoluble in water | 0.05 |
| Sulphate as So4 | 0.1 |
| Lead as Pb | 0.0001 |
| Iron as Fe | 0.001 |
| Ammonical Nitrogen as NH4 HSO4 | 0.15 |
| Synonyms of Sulphamic Acid | SULFAMIC ACID, Sulfamidic acid, Amidosulfonic acid, Jumbo, Sulphamic acid, Amidosulfuric acid, Aminosulfonic acid, Imidosulfonic acid, Sulfaminic acid, Aminosulfuric acid,Sulphamidic acid, Sulfamidsaeure, sulfuramidic acid, Amidoschwefelsaeure, Kyselina sulfaminova |

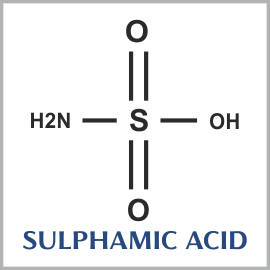
Sulfamic acid, also known as amidosulfonic acid, amidosulfuric acid, aminosulfonic acid, and sulfamidic acid, is a molecular compound with the formula H3NSO3. This colorless, water-soluble compound finds many applications. Sulfamic acid melts at 205 °C before decomposing at higher temperatures to water, sulfur trioxide, sulfur dioxide and nitrogen.
Sulphamic acid can be used for removing scales obtained from water in heating and cooling systems e.g. boilers, condensers, heat exchangers, jackets and coils. Find out applications of sulphamic acid in various industries.
Sulphamic Acid is available in H.D.P.E. bags of net 50 Kgs. Material.Sample packets of 100-200 gms. are supplied on request. Feel free to send us enquiry about sulphamic acid.
Sulphamic Acid is super efficient descaling agent and is used for cleaning a variety of industrial equipment and domestic appliances.
Sulphamic Acid prevents pulp degradation due to temperature at the chlorination and hydrochloride stage. It permits bleaching at higher temperature and lower PH without loss of strength.
Sulphamic Acid removes excess of nitrides used in the diazotization reactions in the manufacturing of dye stuffs and pigments. Nitrides if present in process water of effluents can also be removed by using Sulphamic Acid.
Chlorine gas in water form HOCL which reacts with Sulphamic Acid form N-Chloro-Sulphamic Acid to N-ChloroSufamic Acid more stable and yet has active chlorine, Because of this, Sulphamic Acid is used for stabilizing chlorine in swimming pools and cooling towers.
Metal sulphamate electrolytes values for their high solubility cadmium, cobalt nickle, lead silver and radium sulphamte deposits are bright and dense. Lead sulphamate is used in refining lead when a high quality is desired.
Sulphamic acid is used for sulphation and sulphamation of many organic compounds. Sulphation of aklyl pheno-ethylen oxide condensation products 9for detergents and sulphation of ethoxylated phenol-formaldehyde resins is preferable with Sulphamic Acid. Stronger agents cause unwanted ring sulphomation.
Sulphamic acid finds application in the plastic wherever a curing agents is required which does not have the disadvantage of inorganic acids and which acts faster than organic acids.
No Fumes, no hazards to equipments and personnel.
Rapid Softening, disintegration and dissolving of scales.
By reducing down-time, productivity is increased. by protecting metal and by uniform leaning of the surface equipment life is prolonged and performance made superior. The above information is adequate for commonly encountered problems of descaling.